BioWire Bytes 016 – A Shot at Curing Type 1 Diabetes
Byte-sized biotech
For more than a century, people with type 1 diabetes (T1D) have stayed alive by injecting insulin to manage their blood glucose. But insulin therapy is a treatment, not a cure. In T1D, the immune system wipes out the pancreas’s insulin‑producing beta cells, forcing patients to monitor blood sugar constantly and inject insulin to survive. It’s a relentless routine that manages symptoms but doesn’t restore the body’s natural glucose thermostat.
If we know what causes T1D, are we close to curing it?
On paper, T1D feels solvable. We know the disease cause, and we know the solution: replace the lost beta cells with functioning ones. It’s pretty straightforward. In fact, doctors know that transplanting these cells can free diabetics from insulin shots, as proven by pioneering trials like the Edmonton Protocol, treatment strategies that transplant islet cells into recipients’ livers to stabilize blood sugar.
So, what’s stopping us from curing T1D today if it’s so simple?
In practice, however, this has been anything but simple; the body's immune system acts as the bottleneck by attacking any transplanted cells. While doctors have long known that transplanting healthy beta cells can free patients from insulin shots, treatment strategies that transplant islet cells into recipients must be simultaneously accompanied by immunosuppressants to prevent the body from rejecting the transplanted cells. It would be a fair critique to say this is trading one set of problems for another, as immunosuppressants have nasty side effects. As a result, islet transplants have been limited to only the most severe cases; even the recent FDA-approved islet therapy Lantidra is available to very few patients and still requires heavy immune suppression. The holy grail has been to protect new insulin-making cells from the immune system without immunosuppression. Now, a new strategy is emerging to engineer “stealth” beta cells that hide from the immune system entirely. And in a first-of-its-kind human test, these cloaked cells are showing they can survive and function in a diabetic patient’s body, giving the field of diabetes treatment a literal shot in the arm.
First, if you enjoy these updates, consider subscribing and becoming a part of our growing community!
Hiding Beta Cells from the Immune System
The immune system is constantly patrolling the body and fiercely targets anything it perceives as “foreign” or dangerous. Unfortunately, in type 1 diabetes, the patient’s own beta cells ended up on the immune hit list due to a case of mistaken identity. This means any transplanted replacement beta cells would normally get attacked and destroyed as well. Researchers led by Prof. Per-Ola Carlsson at Uppsala University (with collaborators in Norway and the US) devised a clever genetic disguise to let implanted cells fly under the immune radar. Using CRISPR gene editing and a modified virus, they re-engineered donor islet cells to be “hypoimmune”, essentially invisible to the immune system. First, they deleted the genes for human leukocyte antigen (HLA) class I and II proteins. These HLA molecules are like ID badges that our cells wear; immune T-cells constantly check them to recognize what is “self” and what isn’t. By removing HLA, the team made the transplanted cells much harder for T-cells to identify as foreign invaders (or even as beta cells at all).
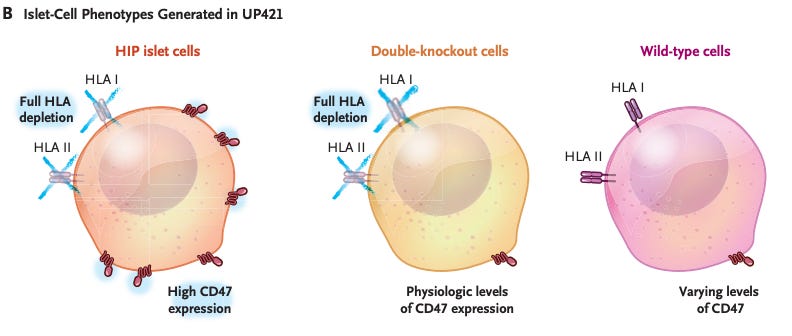
Of course, completely knocking off the cells’ “ID badges” creates another problem: certain immune sentinels called natural killer (NK) cells tend to destroy any cell that lacks HLA (a tactic to fight virus-infected or cancerous cells that often lose HLA to hide). To dodge this, the researchers gave their islet cells an additional layer of security: they added a gene to produce CD47, a protein that basically sends a “don’t eat me” signal to immune cells like macrophages and NK cells. CD47 is normally used by healthy cells (and notoriously, by cancer cells) to tell the immune system to leave them alone. With this two-pronged strategy – HLA gone to avoid T-cell attack, CD47 up to ward off innate immune attack – the engineered beta cells were equipped to survive in a hostile immune environment.
But the real test is how effective this treatment is in the clinic?
First Patient, First Test — and It Worked
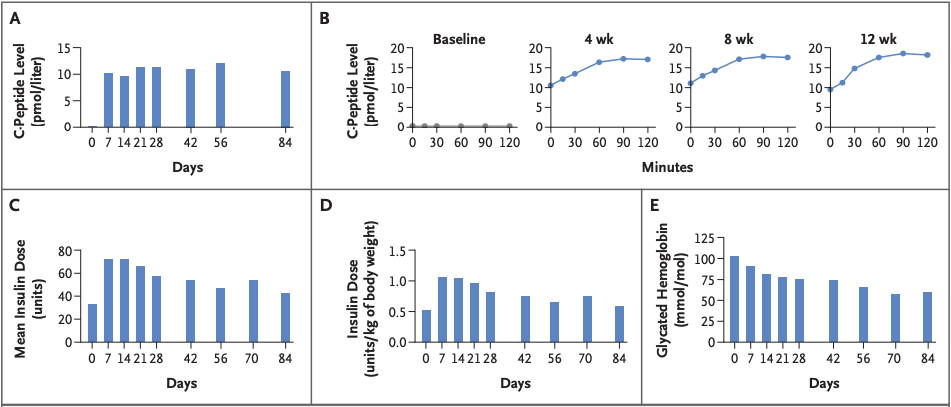
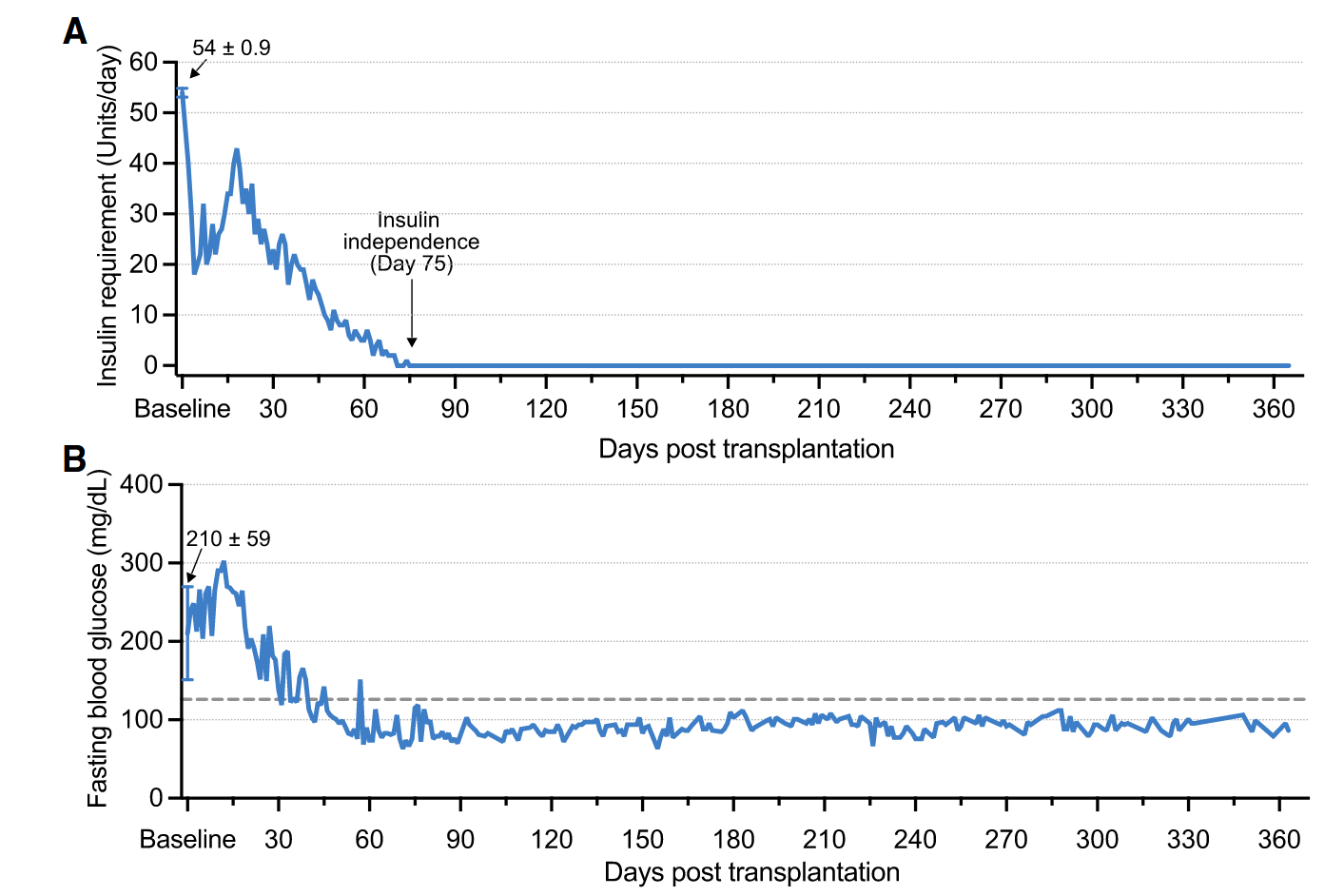
This treatment prototype was put to the test in a first-in-human study. Doctors in Uppsala transplanted the hypoimmune beta cells (derived from a donor pancreas and edited in the lab) into the forearm muscle of a 42-year-old man who had lived with type 1 diabetes for over 35 years (Carlsson et al., 2025). Crucially, they did not give him any immunosuppressive drugs, the key departure from all previous transplants. Would the disguised cells survive without the body attacking them? The early results are extremely encouraging: even 12 weeks after transplant, tests showed no sign of an immune attack on the implanted cells. In fact, the patient’s immune system seemingly didn’t even notice the foreign cells were there.
Better yet, the transplanted beta cells did their job, they began to produce insulin in the patient’s body. Before the transplant, the patient had zero detectable C-peptide (a marker of one’s own insulin production) in his blood. After transplant, C-peptide became detectable and even rose when he ate a meal, indicating the new beta cells were sensing glucose and releasing insulin appropriately. In other words, the graft was functioning like real pancreas tissue, responding to the body’s needs. This was achieved with only a relatively small number of cells transplanted, as the initial trial was designed to test safety more than to cure the disease. The patient still relies on injected insulin, but the experiment proved the concept: engineered cells can evade the immune system and function in a human body. Importantly, no serious side effects occurred in the study. As the principal investigator Dr. Carlsson noted, restoring insulin production without immunosuppression is a “transformative outcome” and a significant step toward an accessible cure. It marks the first time an allogeneic (donor-derived) cell transplant has survived in a person with type 1 diabetes without the usual immune rejection hurdles.
Other related approaches
While all of this is extremely exciting, there are simultaneously other strategies being pursued to replace these insulin-porducing cells in patients. In fact, we’ve covered something similar in a previous post: a new clinical trial where researchers reprogrammed cells from the patient’s abdominal fat into chemically induced pluripotent stem cells (CiPSCs). These CiPSCs were then differentiated into insulin-producing islets and transplanted beneath the abdominal anterior rectus sheath. In this case, the beta cells may not require cloaking from the immune system. Just months after the transplantation, the patient began producing insulin autonomously and achieved stable blood sugar control without the need for exogenous insulin. At the 1-year mark, the patient maintained over 98% time-in-range blood glucose levels with no transplant-related complications, meeting the study’s clinical endpoint (Wang et al., 2024).
This related trial shows another potential cure for T1D with an autologous approach. This strategy may have some tradeoffs though; it requires differentiation of beta cells from patient cells and still may ultimately be destroyed by the immune system without immunosuppressant drugs. Only time will tell what is the best path forward.
An Insulin-Free Future
These milestones are just the beginning, but it has the feeling of an inflection point. “The data represent a significant advancement in the ongoing pursuit of a definitive cure for type 1 diabetes,” said Dr. James Shapiro, the surgeon who pioneered the original islet transplant protocol over two decades ago. Of course, much work lies ahead before this approach can become a widespread therapy. The reported case was a single patient and a low-dose transplant, only about 2–7% of the number of islet cells that a typical diabetic would need to achieve full insulin independence. The next step will be to figure out how many transplanted cells (and perhaps how many rounds of transplantation) are required to completely take over insulin production. Researchers also need to observe how long the cloaked cells can survive and function; so far, results up to 6 months have been promising, with the graft still producing insulin at that point.
Encouragingly, the company behind the cell engineering (Sana Biotechnology) is already working on scaling up the solution. They’re developing a version of these hypoimmune islet cells derived from stem cells, dubbed SC451, which could provide an unlimited supply of beta cells as a one-time treatment for diabetes. The goal, within the next couple of years, is to start clinical trials to test whether a larger dose of such cells can let patients maintain normal blood sugars with no injections and no immunosuppressant drugs. If successful, this would truly be a functional cure for type 1 diabetes – essentially giving patients a brand-new, working set of beta cells that the body accepts as its own.
There is even broader excitement that this immune-evasion cell therapy could extend beyond diabetes. The same hypoimmune “cloak” strategy might one day be applied to transplant other cell types or tissues without rejection, from repairing hearts after a heart attack to treating Parkinson’s with neuron transplants, all without forcing patients to take immune-suppressing medications. But for now, the spotlight is on type 1 diabetes. After a century of insulin shots, the prospect of a once-and-done cell transplant that liberates patients from daily injections has moved a step closer to reality. It’s a rare piece of medical news that’s both scientifically groundbreaking and profoundly human in its promise. If future trials bear out this approach, millions of people living with diabetes may finally get to trade in their syringes and pumps for a brief treatment course of new insulin-producing cells.
These newsletters take significant effort to put together and are totally for the reader's benefit. If you find these explorations valuable, there are multiple ways to show your support:
Engage: Like or comment on posts to join the conversation.
Subscribe: Never miss an update by subscribing to the Substack.
Share: Help spread the word by sharing posts with friends directly or on social media.
References:
Carlsson, P.O., Hu, X., Scholz, H., Ingvast, S., Lundgren, T., Scholz, T., Eriksson, O., Liss, P., Yu, D., Deuse, T. and Korsgren, O., 2025. Survival of Transplanted Allogeneic Beta Cells with No Immunosuppression. New England Journal of Medicine.
Wang, S., Du, Y., Zhang, B., Meng, G., Liu, Z., Liew, S.Y., Liang, R., Zhang, Z., Cai, X., Wu, S. and Gao, W., 2024. Transplantation of chemically induced pluripotent stem-cell-derived islets under abdominal anterior rectus sheath in a type 1 diabetes patient. Cell, 187(22), pp.6152-6164.
https://www.ualberta.ca/en/alberta-diabetes/about/edmonton-procotol.html




An Excellent Article giving type 1 diabetics and their families something to look forward to in the future. It’s nice to know that this research is taking place, and the progress that has been made.