Welcome to 2025, Readers. Let’s be relentless this year!
To kick off this year, I’m thrilled to share what I believe are the Top 5 Biotech Breakthroughs of 2024. Continuing the tradition I began with last year’s review (the 2023 list can be found here), this list highlights the most transformative technologies and innovations that shaped the biotech landscape over the past 12 months. Reflecting on this progress is key to understanding how these advancements will shape the future of healthcare, longevity, and beyond.
This year’s review has a special twist. I had the privilege of coauthoring this review with a friend I made here on Substack, Riccardo Scribano. Riccardo runs an incredible Substack of his own,
, which I highly recommend subscribing to, linked below. Together, we’ve curated this list based on novelty, technological impact, scientific validation, efficacy, and market relevance. To highlight other important innovations and technologies that haven’t quite made this list yet, we’ve also compiled a separate list of Honorable Mentions, which can you read in full on the Substack. I highly recommend checking these out!BioDodo is the side-project of a computational biology PhD student with a keen interest for scientific journalism. Started in September 2024, BioDodo explores the intersection of history, philosophy and the life sciences, making complex technical subjects accessible to everyone.
Now, let’s dive into the breakthroughs that defined 2024 and set the stage for what’s next.
5. New HIV prophylactic: capsid inhibitor prevents 100% of infections in Phase-3 human study
This year's Top 5 biotech breakthroughs start with a treatment that marks a significant advancement in the fight to eradicate HIV worldwide. Only two doses of the viral capsid inhibitor Lenacapavir per year were shown to protect against HIV infection in two large (phase-3) human studies (Bekker et al., 2024, Kelley et al., 2024).
Although antiretroviral treatments allow HIV-seropositive people to live long and healthy lives, HIV continues to affect the lives of millions of people. Between 65.0 million and 113.0 million people have been infected with HIV during the last 40 years, and between 32.9 million and 51.3 million people have lost their lives to the virus.
An HIV vaccine is currently out of sight, with its progress hampered by the biology of the HIV virus, rendering "traditional" vaccinations ineffectual in the long run. The creation of vaccines that produce long-lasting immunity against HIV is significantly hampered by the virus's rapid rate of host mutation, its capacity to disguise its antigens, and their resemblance to human proteins. In contrast to vaccinations, pre-exposure prophylaxis (PrEP) uses mechanisms other than triggering cell-mediated immunity to prevent HIV infection. Prior to this year, PrEP required daily or on-demand administration, which presented a substantial obstacle to their widespread adoption.
In 2022, the FDA and the EMA authorized Lenacapavir, a first-in-class capsid inhibitor, as an antiretroviral treatment. By attaching itself to the viral capsid, the protein envelope that contains the genetic material of the virus, the medication stops new viral particles from forming inside infected cells. Because of its affinity for the capsid, Lenacapavir was also a promising candidate for preventing infections before they occurred. Two phase-3 clinical trials (PURPOSE1 and PURPOSE2) were conducted last year (2024) to assess the effectiveness of twice-yearly Lenacapavir injections in preventing HIV infections in at-risk individuals. In one study, two cases of HIV infection were reported in the Lenacapavir group, whereas no HIV infections were observed in the Lenacapavir group of the other study. Altogether, these outstanding results make Lenacapavir the longest-lasting and most effective PrEP ever tested on humans, which the journal Science has acclaimed as the breakthrough of the year in 2024.
4. Innovation in Induced Pluripotent Stem Cells Reverse type-1 Diabetes in Human Study
Obesity and related conditions, like type 2 diabetes, often dominate discussions about metabolic health. But this year, attention has shifted to a promising breakthrough for individuals with type 1 diabetes, a condition affecting nearly 2 million Americans who depend on daily insulin to manage blood sugar levels. For these patients, the dream of restoring natural insulin production may soon become a reality thanks to advances in induced pluripotent stem cells (iPSCs).
iPSCs are adult cells reprogrammed into a stem-cell-like state, capable of differentiating into various cell types, including insulin-producing islets. For years, researchers have envisioned using iPSCs to create patient-specific islets to restore the body's natural ability to produce insulin, offering a potential cure rather than symptom management. However, reprogramming cells to iPSCs with transcription factors has challenges such as low efficiency, tumorigenicity, and high cost, hindering their translation into clinical treatments. Now, chemically induced pluripotent stem cells (CiPSCs) are addressing these barriers, with the first 1-year results from a groundbreaking first-in-human phase I clinical trial.
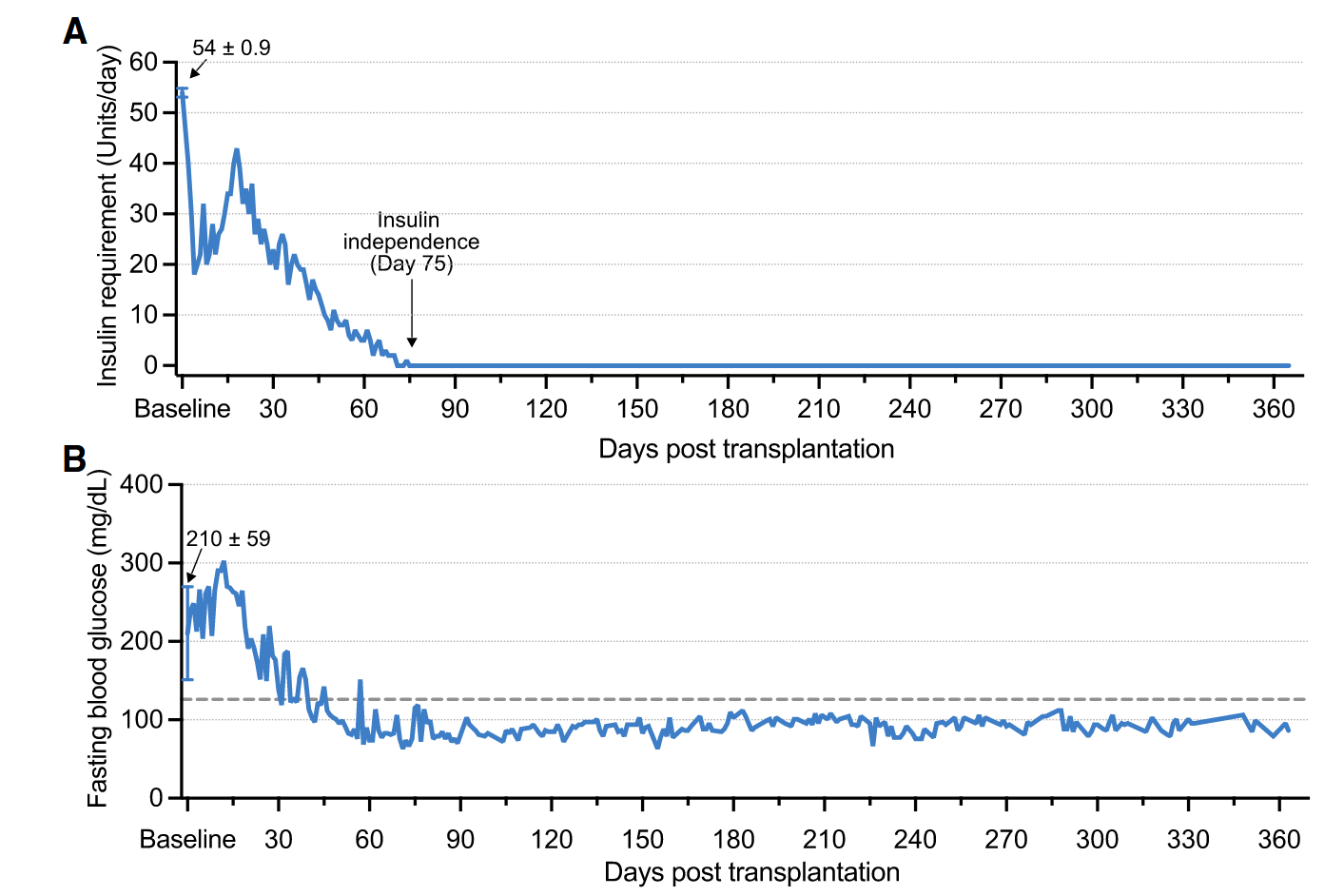
In this trial, researchers reprogrammed cells from the patient’s abdominal fat into chemically induced pluripotent stem cells (CiPSCs)—a safer and more efficient reprogramming approach than traditional genetic methods. These CiPSCs were then differentiated into insulin-producing islets and transplanted beneath the abdominal anterior rectus sheath. Just months after the transplantation, the patient began producing insulin autonomously and achieved stable blood sugar control without the need for exogenous insulin. At the 1-year mark, the patient maintained over 98% time-in-range blood glucose levels with no transplant-related complications, meeting the study’s clinical endpoint (Wang et al., 2024).
This trial marks a turning point for regenerative medicine. Not only does it demonstrate that CiPSC-derived islets can provide long-term insulin independence for type 1 diabetes patients, but it also validates the broader potential of chemically reprogrammed cells. With fewer risks, lower costs, and scalable manufacturing compared to traditional stem cell therapies, CiPSCs could revolutionize treatments for a host of conditions, from neurodegenerative diseases to organ regeneration.
As these therapies progress through clinical trials, their impact on type 1 diabetes could serve as a model for regenerative medicine’s transformative power. CiPSCs represent the culmination of decades of research into personalized medicine, offering a glimpse of a future where even the most chronic diseases might be reversible.
3. Semantic Decoding Translates Thoughts into Action
One of the most science fiction-esque breakthroughs is the advancement in semantic decoding, which focuses on translating human thoughts into text, speech, or action directly from brain activity. This field has seen significant development in both brain-computer interface (BCI) hardware, which detects brain activity, and the advanced machine-learning algorithms capable of decoding these digitized thoughts. But what kind of information have we been able to decode? This includes entire sentences, visual imagery, and intentional movement. Below, we review examples from early academic proof-of-concept studies to technologies currently in clinical trials for medical interventions, showcasing why semantic decoding made it to the top 5 list.
In academic settings, researchers have demonstrated systems that can interpret language, decoding a person’s thoughts as they silently "read" or imagine speaking (Tang et al., 2023; Défossez et al, 2023). Similarly, visual experiences or thoughts have been decoded and reconstructed (Takagi et al, 2023) as shown in the figure below. These approaches leverage deep neural networks to map brain activity patterns—recorded through BCI hardware like functional MRI or high-density EEG—to semantic representations of language or concepts. It is worth mentioning, that these systems were not narrowly limited to pre-learned vocabulary and images but were generalizable to novel sentences and images.

But this is just the academic setting, how about what’s reaching the clinic?
Advanced semantic decoding technology is beginning to be realized in clinical trials for the treatment of disabled individuals. Notably, Elon Musk’s company, Neuralink, has initiated its Phase I Prime Trial by fitting BCI devices directly to patients’ motor cortices. While these devices are highly invasive, they capture high-resolution brain signals, enabling individuals with quadriplegia to operate electronic devices such as smartphones and computers. The trial has announced 2 successful patients implanted with Neuralink’s device, Telepathy. These patients have since showcased the ability to interact with computers to perform various tasks such as composing messages, navigating the internet, and even playing games like chess and high-reflex first-person shooters. A standout moment occurred during an interview when the first patient, Nolan, turned down the music on his computer in real-time via thought, allowing him to better hear the interviewer’s question.
Neuralink isn’t the only player in the rapidly advancing field of semantic decoding and BCIs. Companies like Synchron and Blackrock Neurotech are pioneering medical-grade solutions, with Synchron’s endovascular implants offering a minimally invasive alternative and Blackrock’s NeuroPort Array enabling robotic limb control and thought-based communication in clinical trials. Meanwhile, vision-focused BCIs, like those from Second Sight, aim to restore sight by stimulating the brain’s visual cortex, and consumer-focused companies such as Neurable are developing non-invasive EEG headsets for gaming and virtual reality. These competitors highlight the diversity of approaches and applications, from medical breakthroughs to everyday use cases.
Semantic decoding is advancing rapidly, with medical applications as just the beginning of this transformative technology. While the potential for high-bandwidth interaction with electronics is exciting, it brings nightmarish privacy implications. Just think, if companies and governments don’t respect privacy on computers and smartphones, how will they react to devices with direct access to users’ personal thoughts? It’s a chilling and dystopian scenario, underscoring the need for early discussion and guardrails. As BCI technology becomes less invasive and more ubiquitous, fostering an informed populace will be critical to creating frameworks that ensure privacy and autonomy.
With rapid advances in BCI devices and algorithm efficiency, semantic decoding is poised to become one of the defining technologies of the next decade.
2. Next-Gen AI Models for Drug Discovery
It’s no secret that I’m bullish on AI Drug Discovery. In fact, I published a deep dive here earlier this year on ‘Why AI Will Break Biotech’s Death Spiral’. But it’s already becoming true, with a parabolic uptrend in clinical studies of AI-developed drugs and now some reaching commercialization (Jayatunga et al., 2024). But the technology is still developing, particularly with new models like AlphaFold 3 leading the charge. Building on the groundbreaking success of its predecessors, this next-generation AI model introduced enhanced capabilities for protein interaction prediction, binding affinity estimation, and small molecule docking (Abramson et al., 2024). These advancements have accelerated the design of novel therapeutics, particularly in challenging areas such as protein-protein interactions and antibody optimization.
This year, AlphaFold’s transformative impact was recognized with the Nobel Prize in Chemistry, awarded to the developers for their contributions to structural biology and drug discovery. The technology's ability to predict protein structures with atomic-level accuracy has been likened to the invention of X-ray crystallography, fundamentally changing how we approach biology and medicine.
Unlike earlier versions, AlphaFold 3 integrates multi-modal datasets, combining structural data with functional and dynamic information. This allows researchers to not only predict static protein structures but also model how proteins change shape under physiological conditions. The result is an unprecedented ability to design drugs that precisely target elusive or previously "undruggable" proteins, such as those implicated in neurodegenerative diseases and cancer.
This year saw AlphaFold 3 powerfully applied in real-world contexts. Pharmaceutical companies leveraged the model to identify and optimize new therapeutic candidates in weeks—a process that traditionally took years. For example, it was instrumental in the design of next-generation bispecific antibodies, leading to the FDA approval of the first AI-designed antibody for oncology. Additionally, AI-driven insights have helped identify alternative drug targets for rare diseases, providing hope to underserved patient populations.
The ripple effects of this technology are immense. By reducing costs and timelines, AI models like AlphaFold 3 are democratizing access to drug discovery tools, enabling even small biotech startups to innovate at a scale previously reserved for industry giants. With continuous refinement and integration into end-to-end discovery pipelines, these AI platforms are cementing their role as indispensable tools in modern biopharma.
As the pace of discovery accelerates, the possibilities for personalized and precision medicine grow exponentially. AlphaFold 3 and similar models aren’t just advancing science—they’re reshaping the future of healthcare.
1. GLP-1 Receptor Agonists Advancements and Expanded Applications Are Changing the Trajectory of Obesity and Addiction
One of the most transformative developments in biotech this year has been the advancement and expanded therapeutic applications of GLP-1 receptor agonists and related drugs. Originally developed for type 2 diabetes, these drugs have redefined obesity treatment due to their unparalleled efficacy in promoting weight loss. Remarkably, the U.S. adult obesity rate declined for the first time in at least 50 years—a testament to the profound impact of GLP-1-based therapies. This breakthrough reflects not only the drugs' metabolic effects but also their growing accessibility, bolstered by the advent of oral formulations, which offer a more convenient alternative to injectables.
The year also brought advancements in dual- and triple-agonist therapies, which combine GLP-1 with additional pathways like GIP and glucagon. These next-generation drugs, such as tirzepatide (Zepbound), have demonstrated superior efficacy in clinical trials, achieving even greater weight loss and metabolic improvements than earlier agents like semaglutide (Ozempic) (Rodriguez et al., 2024). Beyond convenience and potency, these therapies are expanding the reach of GLP-1 receptor agonists into previously untapped areas of medicine, exemplifying their versatility as a drug class.

Perhaps most striking is the emerging role of GLP-1 drugs in treating addiction. Once anecdotal, reports of their ability to curb addictive behaviors have now been substantiated by clinical studies, show potential efficacy for alcohol use disorder and opioid use disorder (Chuong et al., 2023, Wang et al., 2024). It is hypothesized these drugs work by influencing brain pathways tied to reward and impulse control, a mechanism with implications that extend far beyond metabolic diseases.
As research progresses, the societal implications of these drugs are becoming increasingly clear. They hold the promise of not only improving individual health outcomes but also reducing the burden of lifestyle-related diseases on healthcare systems worldwide. The potential applications seem almost boundless, making this drug class one of the most exciting areas in biotech today.
Support:
These newsletters take a significant amount of effort to put together and are totally for the benefit of the reader. If you find these explorations valuable, there are multiple ways to show your support:
Engage: Like or comment on posts to join the conversation.
Subscribe: Never miss an update by subscribing to the Substack.
Share: Help spread the word by sharing posts with friends directly or on social media.
References:
Abramson, J., Adler, J., Dunger, J., Evans, R., Green, T., Pritzel, A., Ronneberger, O., Willmore, L., Ballard, A.J., Bambrick, J. and Bodenstein, S.W., 2024. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature, pp.1-3.
Balbach, M., Rossetti, T., Ferreira, J., Ghanem, L., Ritagliati, C., Myers, R.W., Huggins, D.J., Steegborn, C., Miranda, I.C., Meinke, P.T. and Buck, J., 2023. On-demand male contraception via acute inhibition of soluble adenylyl cyclase. Nature communications, 14(1), p.637.
Bekker, L.G., Das, M., Abdool Karim, Q., Ahmed, K., Batting, J., Brumskine, W., Gill, K., Harkoo, I., Jaggernath, M., Kigozi, G. and Kiwanuka, N., 2024. Twice-yearly lenacapavir or daily F/TAF for HIV prevention in cisgender women. New England Journal of Medicine, 391(13), pp.1179-1192.
Chuong, V., Farokhnia, M., Khom, S., Pince, C.L., Elvig, S.K., Vlkolinsky, R., Marchette, R.C., Koob, G.F., Roberto, M., Vendruscolo, L.F. and Leggio, L., 2023. The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission. JCI insight, 8(12).
Défossez, A., Caucheteux, C., Rapin, J., Kabeli, O. and King, J.R., 2023. Decoding speech perception from non-invasive brain recordings. Nature Machine Intelligence, pp.1-11.
Gomes, A.A., Santos, N.C., Rosa, L.R., Borges, R.J., Fontes, M.R., Hamil, K.G., O’Rand, M.G. and Silva, E.J., 2023. Interactions of the male contraceptive target EPPIN with semenogelin-1 and small organic ligands. Scientific reports, 13(1), p.14382.
Heymsfield, S.B., Coleman, L.A., Miller, R., Rooks, D.S., Laurent, D., Petricoul, O., Praestgaard, J., Swan, T., Wade, T., Perry, R.G. and Goodpaster, B.H., 2021. Effect of bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity: a phase 2 randomized clinical trial. JAMA network open, 4(1), pp.e2033457-e2033457.
Jayatunga, M.K., Ayers, M., Bruens, L., Jayanth, D. and Meier, C., 2024. How successful are AI-discovered drugs in clinical trials? A first analysis and emerging lessons. Drug Discovery Today, p.104009.
Kelley, C.F., Acevedo-Quiñones, M., Agwu, A.L., Avihingsanon, A., Benson, P., Blumenthal, J., Brinson, C., Brites, C., Cahn, P., Cantos, V.D. and Clark, J., 2024. Twice-Yearly Lenacapavir for HIV Prevention in Men and Gender-Diverse Persons. New England Journal of Medicine.
Kim, P., Sanchez, A.M., Penke, T.J., Tuson, H.H., Kime, J.C., McKee, R.W., Slone, W.L., Conley, N.R., McMillan, L.J., Prybol, C.J. and Garofolo, P.M., 2024. Safety, pharmacokinetics, and pharmacodynamics of LBP-EC01, a CRISPR-Cas3-enhanced bacteriophage cocktail, in uncomplicated urinary tract infections due to Escherichia coli (ELIMINATE): the randomised, open-label, first part of a two-part phase 2 trial. The Lancet Infectious Diseases, 24(12), pp.1319-1332.
Nunn, E., Jaiswal, N., Gavin, M., Uehara, K., Stefkovich, M., Drareni, K., Calhoun, R., Lee, M., Holman, C.D., Baur, J.A. and Seale, P., 2024. Antibody blockade of activin type II receptors preserves skeletal muscle mass and enhances fat loss during GLP-1 receptor agonism. Molecular Metabolism, 80, p.101880.
Rodriguez, P.J., Cartwright, B.M.G., Gratzl, S., Brar, R., Baker, C., Gluckman, T.J. and Stucky, N.L., 2024. Semaglutide vs tirzepatide for weight loss in adults with overweight or obesity. JAMA Internal Medicine.
Shi, R., Wolgemuth, D.J. and Georg, G.I., 2025. Development of the retinoic acid receptor alpha-specific antagonist YCT-529 for male contraception: A brief review. Contraception, p.110809.
Tang, J., LeBel, A., Jain, S. and Huth, A.G., 2023. Semantic reconstruction of continuous language from non-invasive brain recordings. Nature Neuroscience, 26(5), pp.858-866.
Wang, W., Volkow, N.D., Wang, Q., Berger, N.A., Davis, P.B., Kaelber, D.C. and Xu, R., 2024. Semaglutide and Opioid Overdose Risk in Patients With Type 2 Diabetes and Opioid Use Disorder. JAMA Network Open, 7(9), pp.e2435247-e2435247.
Wang, S., Du, Y., Zhang, B., Meng, G., Liu, Z., Liew, S.Y., Liang, R., Zhang, Z., Cai, X., Wu, S. and Gao, W., 2024. Transplantation of chemically induced pluripotent stem-cell-derived islets under abdominal anterior rectus sheath in a type 1 diabetes patient. Cell.


















What a year! I feel like we're just getting started.
Im getting my first, in a series, of qEEGs next week. Im counting on technology associated with brain mapping. I wish I knew everything before proceeding but I don't. I can only digest so much data by Monday😊