Happy Sunday Morning, Readers. Let’s prepare to be relentless this week!
The topics this week:
The Next Generation of Weight Loss Drugs Will Be a Temptation for Everyone.
The First Oral GLP-1 for Obesity Nears Market Readiness
Thirty Years of Data Point to Diets That Preserve Health in Aging
NIH Moves to Prioritize Human-Based Research Models
The reproducibility crisis exists even in papers published with common techniques and methods
Metformin Shows Promise for Knee Osteoarthritis in Overweight Patients
First, if you enjoy these updates, consider subscribing and becoming a part of our growing community!
Next Generation Weight Loss Drugs Will Be a Temptation for Everyone
Losing weight is notoriously challenging, particularly in a time with addictive foods and overly sedentary lifestyles. Moreover, it requires depriving your body of external calories, below what is metabolically required to sustain yourself, to create a deficit and draw energy from reserve tissue, e.g., stored fat and muscle. Sustaining this deficit leads to gradual weight loss, at least until a plateau is reached, when the new, lower calorie intake matches the calories required for maintenance. In an ideal world, this weight loss would come exclusively from fat reserves. How great would that be, right? Unfortunately, the reality is quite a bit different. It’s nearly impossible to lose exclusively fat during weight loss; this is accompanied by muscle loss as well. This muscle loss is partly why people experience significant reductions in strength during prolonged periods of dieting.
The degree to which muscle is lost during dieting depends on a variety of factors, such as the severity of the caloric deficit and the diet macronutrient composition, where more severe dieting and lower protein intake often result in greater muscle loss. This issue highlights a fundamental limitation of the current generation of weight-loss drugs like semaglutide (Ozempic/Wegovy) and tirzepatide (Zepbound/Mounjaro): individuals taking these products often severely reduce caloric intake and, as a result, lose a disproportionate amount of muscle mass. This reduction of muscle mass is a significant challenge, particularly for the aging population, where it’s a key predictor of longevity and all-cause mortality. This is the motivation for the next generation of weight loss drugs: achieve weight loss results similar or better than GLP-1-related drugs while prioritizing a higher quality of weight loss, i.e., lose fat and maintain muscle. To that end, several companies, including Regeneron have active clinical studies to test drugs for improving this weight loss quality.
Recent interim results from Regeneron Pharmaceuticals' ongoing Phase 2 COURAGE trial suggest a promising advance to these weight-loss therapies, particularly improving body composition. The trial is investigating the combination of the popular GLP-1 receptor agonist semaglutide with trevogrumab, an anti-myostatin antibody, and optionally with garetosmab, an anti-activin A antibody. This novel approach targets the significant muscle loss associated with GLP-1 therapies.

The interim findings revealed some interesting data at the 26-week mid-point of the overall 52-week study. In the control GLP-1 only treatment, approximately 35% of the weight reduction from semaglutide alone was lean mass loss. However, the combination therapies dramatically preserved muscle while further enhancing fat loss. Participants receiving semaglutide combined with trevogrumab only lost 17% of total weight from muscle mass, suggesting the combo preserved around 50% more lean mass compared to semaglutide alone. Even more incredible, when garetosmab was added, only 6.6% lean muscle mass was lost (80% improvement in preservation). Moreover, the triple combination led to significantly greater overall body weight reduction, predominantly from fat loss, achieving a more desirable quality of weight loss and resulting body composition.
These results are in line with prior findings from Regeneron in a non-human primate study, where the dual combo therapy prevented nearly all muscle loss, and the triple-combo therapy actually resulted in additional muscle gain while providing even greater degrees of fat loss. Could you imagine this: taking a treatment that results in fat loss and muscle gain? If this were affordable, I think half the country would be trying to get their hands on it.
Are there any risks? While the combination therapies showed increased tolerability issues, especially the triple regimen, they are still fairly minor it appears. More importantly, these results underscore the potential to significantly improve the quality of weight loss therapies. Regeneron's innovative approach could substantially impact obesity management by enabling weight reduction that conserves muscle mass, potentially enhancing long-term health outcomes and reducing obesity-related comorbidities. The full results, anticipated later this year, will be exciting to see.
The First Oral GLP-1 for Obesity Nears Market Readiness
Eli Lilly’s Orforglipron has become the first oral small-molecule GLP-1 receptor agonist to successfully complete a Phase 3 trial. Why is this a big deal when we are constantly hearing about weight-loss drugs like Mounjaro and Wegovy? These drugs are typically delivered via injection, creating a significant barrier to entry. There had previously been challenges formulating and delivering these drugs as an oral-solid dose. That is until now.
In the ACHIEVE-1 study, adults with type 2 diabetes receiving Orforglipron experienced hemoglobin A1c reductions of 1.3% to 1.6% from a baseline of 8.0%, versus 0.1% with placebo, over 40 weeks. The highest dose also produced an average weight loss of 7.3 kg (16.0 lbs), equivalent to 7.9% of body weight, despite participants not reaching a weight plateau by the study’s end.
More than 65% of participants on the highest dose achieved an A1c below 6.5%, the ADA’s threshold for diabetes remission. Safety outcomes aligned with known GLP-1 class effects: gastrointestinal events were the most frequent, including diarrhea (up to 26%), nausea (up to 18%), and constipation (up to 17%). No hepatic safety signals were observed, a key advantage after Pfizer’s oral GLP-1 candidate was discontinued due to liver toxicity.
The oral formulation offers advantages in accessibility and adherence. Orforglipron requires no food or water restrictions, avoids refrigeration, and can be manufactured at scale. Lilly plans to pursue regulatory approval for weight management by the end of 2025, with a separate diabetes indication submission in 2026. Ongoing Phase 3 trials under the ATTAIN program will assess its efficacy in patients without diabetes but with overweight or obesity, potentially broadening its clinical and commercial impact.
With its early success, orforglipron may represent a pivotal shift in obesity therapeutics—a GLP-1 mimetic in pill form that retains class efficacy while expanding reach through oral delivery.
Thirty Years of Data Point to Diets That Preserve Health in Aging
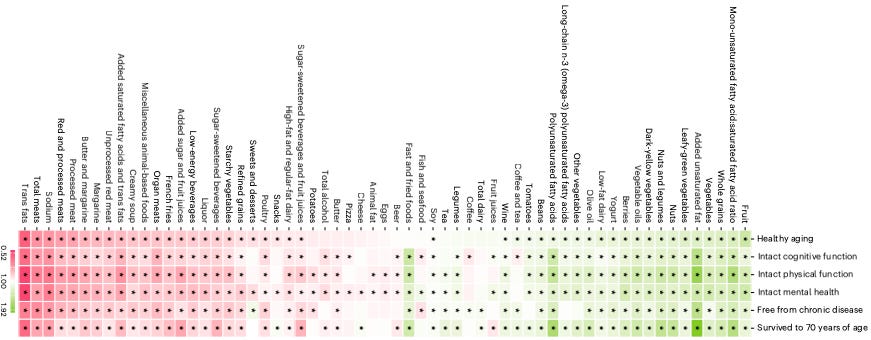
A large prospective study published in Nature Medicine evaluated the long-term impact of dietary patterns on multidimensional healthy aging across two major U.S. cohorts: the Nurses’ Health Study and the Health Professionals Follow-Up Study (Tessier et al., 2025). Over 105,000 participants were followed for up to 30 years, with healthy aging defined by survival to age 70 free of major chronic diseases and with intact cognitive, physical, and mental health.
Among eight dietary patterns assessed, the Alternative Healthy Eating Index (AHEI) was most strongly associated with healthy aging. Participants in the highest quintile of AHEI adherence had an 86% greater chance of healthy aging at age 70 (OR = 1.86, 95% CI = 1.71–2.01), and more than twice the odds when the threshold was shifted to age 75 (OR = 2.24, 95% CI = 2.01–2.50). Diets high in fruits, vegetables, whole grains, unsaturated fats, nuts, legumes, and low-fat dairy were positively associated with aging metrics, while higher intakes of red and processed meat, trans fats, and sodium were linked to worse outcomes.
The study also found that ultra-processed food (UPF) consumption had a negative association with all domains of healthy aging. Individuals in the highest UPF quintile had 32% lower odds of achieving the composite healthy aging phenotype (95% CI = 27–37%). While all evaluated patterns showed some benefit, those integrating moderate animal product intake (e.g., AHEI, DASH, Mediterranean diets) generally outperformed strictly plant-based patterns like the hPDI.
Overall, these findings point to dietary quality as an important determinant of aging outcomes. More importantly, they provide actionable advice to prioritize whole, minimally processed foods to extend both healthspan and lifespan.
NIH Moves to Prioritize Human-Based Research Models
In a major policy shift, the National Institutes of Health (NIH) has announced plans to significantly expand the use of human-based research technologies while reducing reliance on animal models. This initiative parallels the FDA’s recent movement toward minimizing animal testing, such as the elemination of antibody safety studies in animals. This further aims to reorient biomedical research around more translationally relevant platforms. The agency will establish a new Office of Research Innovation, Validation, and Application (ORIVA) to coordinate and scale the development of non-animal approaches across its research portfolio.
The new strategy emphasizes organoids (miniature tissues that emulate key organ features), tissue chips (such as lung-on-a-chip), computational models of human biology, and population-level real-world data. These tools, the NIH argues, offer higher fidelity for modeling human disease, variability, and drug responses, especially in areas where traditional animal models have shown translational limitations, such as neurodegenerative and oncologic diseases.
ORIVA will be tasked with funding, validating, and integrating these technologies, while also mitigating entrenched biases in grant review processes. NIH will expand training programs, adjust evaluation criteria to prioritize human relevance, and begin public reporting on shifts in funding allocations away from animal-based research. Importantly, non-animal methods will no longer be considered secondary or supplemental, but will be evaluated for their standalone suitability and translational accuracy.
This initiative signals a structural transformation in how the U.S. federal research apparatus approaches model selection. Rather than supplanting animal research outright, NIH’s move is designed to broaden methodological scope, incorporating human-mimetic systems as primary discovery tools where appropriate. It is a formal recognition that biomedical innovation increasingly depends on platforms that reflect human biology from the outset.
The reproducibility crisis exists even in papers published with common techniques and methods.
Brazil conducted a reproducibility study in the biomedical area. It was a large-scale effort with over 200 scientists across 56 laboratories, focusing on commonly used methods like cell metabolism assays, genetic material amplification, and rodent maze tests (Amaral et al., 2025). The results were sobering. Across 47 published experiments re-tested using the MTT assay, RT-PCR, and elevated plus maze (EPM), replication success rates varied from 15% to 45%, depending on the metric used. Median replication rate across all criteria was 26%.
Among the most revealing findings: 94% of original effect sizes were larger than their corresponding replication effects. Coefficients of variation were also markedly lower in original studies, by a factor of 2.5 on average, which should raise concerns about underreported variability, overprecision, or methodological inconsistencies in the original literature.
Protocol fidelity was another major challenge. Deviations from preregistered methods were present in 91% of replications. Why? Missing information in original papers, infrastructure gaps, supplier delays, and experimental failures. Even with multiple rounds of protocol refinement and external validation, nearly one-third of replications were excluded due to protocol issues, low sample sizes, or invalid experimental units. Many labs struggled to interpret or implement core methodological elements such as experimental unit definition. This should be a reminder that terminological ambiguity remains a persistent threat to replication.
Despite these obstacles, the project provides a rare, structured snapshot of real-world reproducibility in academic biomedical labs. Unlike prior efforts focused on high-impact or cancer-specific papers, this study drew from a random, method-stratified sample of life sciences research. While replication outcomes were often poor, inter-lab agreement was generally higher than between original studies and replications, pointing to publication bias and selective reporting as upstream drivers of variability.
This effort is just one of many data points highlighting the urgent need for standardized experimental design, clearer methodological reporting, and institutional support for replication infrastructure.
Metformin Shows Promise for Knee Osteoarthritis in Overweight Patients
A randomized clinical trial published in JAMA evaluated whether metformin, a first-line diabetes drug, can alleviate knee pain in patients with osteoarthritis and overweight or obesity (Pan et al., 2025). The study enrolled 107 participants with symptomatic knee osteoarthritis and a BMI ≥25, assigning them to either metformin (2000 mg/day) or placebo for six months. Pain was assessed using a 100-mm visual analog scale (VAS), where higher scores indicate more severe pain.
At six months, patients in the metformin group reported a mean pain reduction of 31.3 mm, compared to 18.9 mm in the placebo group. The between-group difference of −12.4 mm (P=0.01) corresponds to a moderate effect size (0.43), just below the generally accepted minimal clinically important difference of 15 mm. Adverse events were infrequent, with diarrhea and abdominal discomfort being the most commonly reported.
These results may mean that metformin has potential as an adjunct therapy for knee osteoarthritis in overweight individuals, offering anti-inflammatory benefits beyond glycemic control. However, the modest sample size and short trial duration underscore the need for larger, longer-term studies before metformin can be routinely recommended for this indication.
These newsletters take significant effort to put together and are totally for the reader's benefit. If you find these explorations valuable, there are multiple ways to show your support:
Engage: Like or comment on posts to join the conversation.
Subscribe: Never miss an update by subscribing to the Substack.
Share: Help spread the word by sharing posts with friends directly or on social media.
References:
https://newsroom.regeneron.com/news-releases/news-release-details/interim-results-ongoing-phase-2-courage-trial-confirm-potential
https://www.nature.com/articles/d41586-025-01266-x
https://www.nih.gov/news-events/news-releases/nih-prioritize-human-based-research-technologies
Brazilian Reproducibility Initiative, Amaral, O.B., Carneiro, C.F.D., Neves, K., Sampaio, A.P.W., Gomes, B.V., Abreu, M.B.D., Tan, P.B., Mota, G.P.S., Goulart, R.N. and Fernandes, N.R.D.S., 2025. Estimating the replicability of Brazilian biomedical science. bioRxiv, pp.2025-04.
Pan, F., Wang, Y., Lim, Y.Z., Urquhart, D.M., Estee, M.M., Wluka, A.E., Wolfe, R. and Cicuttini, F.M., 2025. Metformin for knee osteoarthritis in patients with overweight or obesity: a randomized clinical trial. JAMA.
Tessier, A.J., Wang, F., Korat, A.A., Eliassen, A.H., Chavarro, J., Grodstein, F., Li, J., Liang, L., Willett, W.C., Sun, Q. and Stampfer, M.J., 2025. Optimal dietary patterns for healthy aging. Nature medicine.